How Varroa Mites Changed Modern Beekeeping
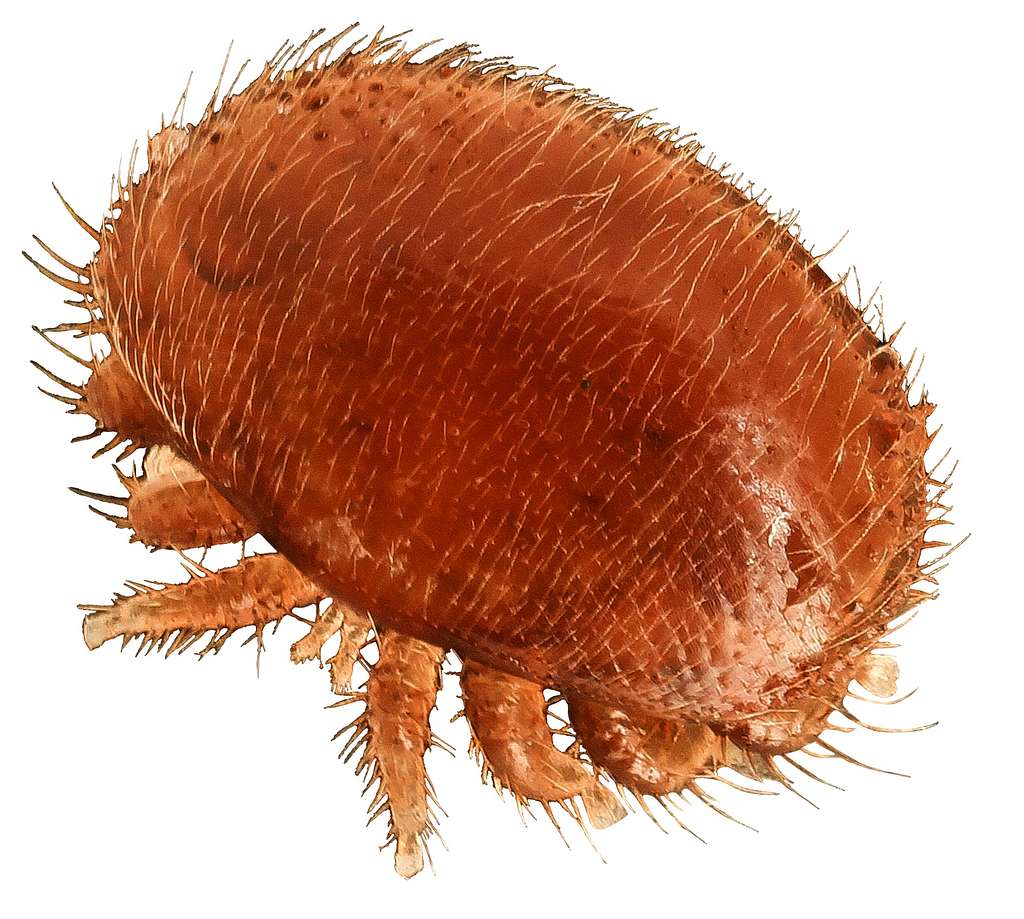
September 25, 1987. A Wisconsin beekeeper named Gary Oreskovic discovered something on his Florida colonies that would fundamentally restructure North American beekeeping. Small reddish-brown mites, about the size of a pinhead, clustered on his bees. Within weeks, 19 of Florida's 67 counties reported the same parasite. Within two years, it appeared in 19 states.
The mite was Varroa destructor. The name translates to "destructive Varroa," and it lived up to the billing.
Beekeepers watched their hives collapse. Bees emerged from cells with deformed wings, crumpled bodies, shortened lifespans. Colonies that looked healthy in August died mysteriously by October. The mortality wasn't gradual. It was catastrophic. Some operations lost 60-80% of their hives in a single season.
European beekeepers had been fighting Varroa since the 1970s, developing treatments and management strategies through painful trial and error. North American beekeepers had a decade to prepare, to watch the disaster unfold overseas and plan accordingly. It didn't help much. When Varroa arrived, it moved faster than expected, hit harder than predicted, and required more intervention than anyone wanted to admit would be permanent.
Before Varroa, beekeeping was something you could do casually. You might open your hives a few times per season, harvest honey in fall, maybe feed sugar syrup if winter stores looked light. Hive losses ran 10-15% annually, mostly from starvation, poor queens, or weather. Beekeepers accepted these losses as normal attrition.
After Varroa, casual beekeeping died. The mites demanded constant attention, regular monitoring, timely treatments, and acceptance that chemical intervention had become as fundamental to colony management as providing adequate food stores. Beekeeping transformed from agriculture into an ongoing battle against an invasive parasite that, left unchecked, would kill every colony within 2-3 years.
A Parasite That Wasn't Supposed To Be There
Varroa destructor evolved on Asian honey bees (Apis cerana) in southern Asia, probably over millions of years. The relationship reached equilibrium. Asian bees developed behaviors that kept mite populations manageable. They detected infested brood cells and removed the pupae before mites could reproduce. They groomed mites off each other. Their shorter development time gave mites less opportunity to multiply in brood cells.
European honey bees (Apis mellifera), which evolved in Europe and Africa, never encountered Varroa. They had no evolutionary history with this parasite, no defenses, no tolerance. When humans started moving European honey bees into Asia for honey production and crop pollination in the early 1900s, they created an encounter that nature hadn't scheduled.
The host shift took time. Varroa was first described scientifically in 1904, found on Asian bees in Java. But it remained primarily an Asian bee parasite for decades. Exactly when it made the successful jump to European bees remains unclear, but by the 1960s, European bee colonies in Asia started showing heavy infestations.
The rest followed predictably. Beekeepers moved bees internationally. Varroa hitchhiked. Europe saw its first infestations in the early 1970s. South America followed. By 1987, North America's turn arrived, probably through illegally imported queens from South America based on genetic analysis.
What made Varroa so destructive wasn't just the direct parasitism. The mites feed on bee hemolymph (essentially bee blood), weakening individual bees and shortening lifespans. But that's almost secondary damage. The real devastation came from Varroa's role as a disease vector.
The mites act like dirty hypodermic needles, piercing bee exoskeletons and injecting viruses as they feed. Deformed wing virus, acute bee paralysis virus, Israeli acute paralysis virus, and others. These viral diseases existed in bee populations before Varroa, but at low, manageable levels. Varroa transmission turned minor viral presence into epidemic-level infections that killed entire colonies.
A colony with high Varroa loads in late summer faces compounding problems. The mites weaken adult bees, reducing their lifespan from 6 weeks to 3-4 weeks. They damage developing bees in brood cells, producing workers with missing wings or neurological problems. The viruses they vector cause additional mortality and disability. By the time winter arrives, the colony lacks the population and health to survive cold weather.
Untreated colonies in temperate climates typically died within 2-3 years of initial infestation. In warmer climates like Florida, colonies could collapse in under a year. The timeline varied, but the outcome didn't. Without intervention, Varroa killed bee colonies. Every time.
The First Wave of Solutions and Failures
When Varroa first arrived, beekeepers tried everything. Folk remedies, essential oils, natural acids, screened bottom boards, drone brood trapping, small-cell foundation. Some methods showed marginal effectiveness. None solved the problem.
The screened bottom board theory gained particular traction. The idea was that mites groomed or fallen off bees would drop through a screen at the hive bottom and couldn't crawl back up. Testing showed that maybe 10-15% of mites fell through screens naturally. The other 85-90% stayed on bees or in brood cells. Screened bottom boards helped with hive ventilation, but they didn't control Varroa at levels that prevented colony collapse.
Small-cell foundation advocates argued that bees built on smaller cell size (4.9mm instead of standard 5.4mm) would somehow resist Varroa better. The theory suggested that tighter cells made mite reproduction harder. Years of testing found no significant difference in Varroa reproduction rates between cell sizes. The small-cell movement persisted anyway, driven more by philosophy than data.
Essential oils, particularly thymol-based treatments, showed actual effectiveness. Thymol disrupted mite reproduction and caused some adult mite mortality. But it was temperature-sensitive, requiring specific application timing and weather conditions. Used incorrectly, it did nothing. Used correctly, it helped but didn't eliminate mite populations.
The beekeeping community split into camps. Some accepted chemical treatments as necessary. Others rejected chemicals entirely, believing natural selection would eventually produce Varroa-resistant bees if beekeepers just stopped treating and let weak colonies die. This "treatment-free" approach produced passionate advocates and massive colony losses.
The treatment-free philosophy had logical appeal. Selective pressure should favor resistance. Letting susceptible colonies die should concentrate resistant genetics in surviving populations. But the timeline for natural selection to work proved slower than the mite reproduction rate. Most treatment-free operations experienced 60-80% annual losses, replacing colonies faster than resistance could build.
A few isolated populations did show resistance. Feral bee colonies in isolated locations developed some tolerance after massive die-offs killed most susceptible genetics. Some managed operations using specific genetic lines (Russian bees, certain hygienic traits) achieved lower losses. But these remained exceptions rather than solutions available to typical beekeepers.
The chemical treatments that worked came with problems. Fluvalinate (Apistan) was the first widely available miticide, introduced in the early 1990s. It worked brilliantly for about five years. Then mites developed resistance. Coumaphos (CheckMite+) followed, with the same pattern. Work well, develop resistance, become ineffective.
Beekeepers found themselves on a chemical treadmill. Each new treatment worked until it didn't. Rotating between chemicals slowed resistance development but didn't prevent it. The mites reproduced every 10 days during brood-rearing season. That's roughly 15-20 generations per year. Evolution operates quickly at that reproduction rate.
The chemical treatments also introduced honey contamination concerns. Miticides used during honey production could leave residues in the harvest. The FDA set tolerance levels. Some beekeepers violated them accidentally through poor timing or excessive application. The organic honey market rejected any hives treated with synthetic miticides, creating a premium market that most beekeepers couldn't access.
The Management Mindset That Replaced the Harvest Mindset
Before Varroa, beekeeping calendars organized around honey flows. You prepared hives for spring buildup, managed swarm prevention during peak colony growth, extracted honey after major flows, and prepared colonies for winter. Pest and disease management existed but remained secondary to production concerns.
After Varroa, the calendar reorganized around mite control. You monitored mite levels in spring, treated if thresholds exceeded, monitored again before main honey flow, decided whether to treat or wait until after harvest, monitored in late summer (the critical period), treated aggressively before winter bees developed, monitored in winter, and started over.
Honey production became something that happened between mite treatments rather than the primary purpose of keeping bees. Commercial beekeepers particularly felt this shift. Operations that once made money primarily from honey sales found that pollination fees for crop services became the main revenue stream, with honey as secondary income.
The monitoring itself required new skills and equipment. Beekeepers learned to recognize mite damage symptoms: deformed wings, parasitic mite syndrome, irregular brood patterns. They adopted sampling methods: alcohol washes, sugar rolls, sticky board counts. They calculated economic thresholds, the mite level at which treatment costs less than projected colony losses.
A 3% mite infestation rate (3 mites per 100 bees) became the rough threshold for concern. Above that level, mite populations typically exploded during late summer, leading to winter colony loss. Below it, colonies might survive without immediate treatment. But "might survive" offered less certainty than beekeepers wanted.
The timing of treatments mattered enormously. Treating too early wasted product and left colonies vulnerable during critical late-season periods. Treating too late meant mites had already damaged the winter bees, which needed to survive 4-6 months rather than the typical 6-week summer lifespan. Getting timing right required understanding mite reproduction cycles, bee development patterns, and local climate factors.
Commercial operations refined this to a science. They tracked mite levels across hundreds or thousands of colonies, treated on schedules calibrated to regional conditions, and accepted 30-50% annual losses as the new normal. The economics worked because pollination contracts paid enough to cover replacement costs and mite management expenses.
Hobbyist beekeepers struggled more. The monitoring equipment, chemical costs, and learning curve exceeded what many expected when they started keeping bees. "I just wanted some honey for my family" became a refrain from frustrated beekeepers who discovered they'd signed up for ongoing pest management instead of casual agriculture.
The mental shift from harvester to manager affected how beekeepers viewed their colonies. Before Varroa, a strong colony that produced surplus honey represented success. After Varroa, a colony that survived the year with manageable mite levels while still producing some honey became the victory. The bar lowered because the challenge increased.
How Treatment Options Evolved and Stagnated
The miticide arsenal expanded gradually through the 1990s and 2000s, but never as fast as beekeepers needed. Each new treatment offered hope, worked for a while, then lost effectiveness as resistance developed or application challenges emerged.
Organic acids entered the treatment landscape as alternatives to synthetic chemicals. Formic acid, oxalic acid, and lactic acid all showed mite-killing properties. They offered advantages: no resistance development (mites can't evolve resistance to acids that work through physical mechanisms), no honey contamination (acids used by bees naturally), and relatively low cost.
But acids came with application difficulties. Formic acid required specific temperature ranges to work safely. Too hot, and it killed bees along with mites. Too cold, and it didn't vaporize properly to penetrate hive spaces. The commercial applicators helped, but timing application windows around weather remained tricky.
Oxalic acid worked exceptionally well, but only when no capped brood was present. Mites hiding in brood cells escaped exposure. This meant oxalic treatments worked in late fall/early winter when colonies stopped raising brood, or required repeated applications during brood-rearing season to catch mites as they emerged from cells.
Beekeepers developed treatment rotation strategies. Use oxalic acid in winter. Apply formic acid in early spring. Use amitraz (Apivar) if needed during summer. Hit hard with oxalic again in fall. Rotate to prevent resistance. Monitor constantly to know when treatment was actually necessary versus just preventive.
The "soft chemicals" versus "hard chemicals" debate emerged. Soft chemicals (organic acids, essential oils, biological controls) aligned with natural beekeeping philosophies but often worked less reliably than synthetic miticides. Hard chemicals (fluvalinate, coumaphos, amitraz) killed mites more consistently but risked resistance and contamination.
Most successful beekeepers ended up using both. Soft chemicals for regular management, hard chemicals when mite populations exploded beyond soft treatment capacity. The purists on either end (only organic acids, or only synthetic miticides) generally experienced either higher losses or higher treatment costs than integrated approaches.
Research into biological controls continued through this period. Certain fungi (Metarhizium, Beauveria) showed promise in laboratory settings for killing mites. Field application proved more difficult. Getting fungal spores to contact mites inside hive conditions while not affecting bees required delivery systems that never quite materialized commercially.
The treatment landscape stabilized into a handful of effective options rather than the breakthrough silver bullet everyone hoped for. Beekeepers accepted that Varroa management meant ongoing intervention using multiple tools and strategies, not a one-time solution that made the problem disappear.
The Selective Breeding Programs That Showed Promise
If chemicals couldn't solve the Varroa problem permanently, maybe genetics could. Bee breeding programs targeting Varroa resistance and tolerance began in the 1990s, accelerated in the 2000s, and by the 2020s produced measurable results.
The USDA Russian honey bee program imported bee genetics from the Russian Far East, where colonies had coexisted with Varroa since the 1940s-50s. These populations showed better survival under mite pressure than typical European bee stocks. Initial imports occurred in 1997. Distribution to beekeepers for testing began in the early 2000s.
Russian bees demonstrated several helpful traits. They showed stronger hygienic behavior, removing mite-infested brood more readily than other stocks. They reduced drone brood production (mites preferentially reproduce in drone cells). Their grooming behavior removed more mites from adult bees. Used together, these traits reduced mite population growth.
But Russian bees came with trade-offs. They built up colonies more slowly in spring, sometimes too late for early honey flows. They showed different temperament, sometimes more defensive than beekeepers preferred. They required different management approaches than traditional Italian or Carniolan bees most beekeepers were familiar with.
Hygienic behavior became a major breeding focus across multiple programs. Bees with strong hygienic traits detected diseased or parasitized brood and removed it from the hive before problems spread. The VSH trait (Varroa Sensitive Hygiene, originally called Suppressed Mite Reproduction or SMR) specifically targeted mite-infested cells.
Testing for hygienic behavior involved freeze-killing patches of brood and measuring how quickly bees removed the dead brood. Fast removal (under 48 hours) indicated strong hygienic tendencies. Breeders selected queens from colonies showing the best hygienic scores and bred them to drones from similarly selected colonies.
The results appeared gradually. Colonies from hygienic breeding lines showed 20-30% lower mite populations compared to unselected stock under the same conditions. This wasn't complete resistance, but it pushed back against mite population growth enough that treatment requirements decreased or timing became less critical.
Some feral populations developed surprising tolerance. Studies of feral colonies in isolated forests (Arnot Forest in New York, isolated woodlands in parts of the South) found surviving populations that maintained themselves despite Varroa presence. These bees showed smaller colony sizes, different seasonal patterns, and various behavioral adaptations that allowed coexistence with mites.
The Arnot Forest bees particularly intrigued researchers. After massive die-offs in the late 1990s killed most feral colonies, survivors rebuilt stable populations. Testing revealed they had shorter development times, more frequent brood breaks, stronger grooming behavior, and smaller colony sizes that might limit mite reproduction. Whether these traits could transfer to managed colonies remained an active research question.
Commercial queen breeding operations slowly incorporated resistance traits. By the 2020s, you could purchase queens advertised as "hygienic stock," "mite-resistant genetics," or "VSH lineage." The traits weren't magic bullets, colonies still needed mite management, but they reduced treatment intensity and improved survival rates.
The challenge was that most beekeepers bought cheap queens and replaced them frequently rather than investing in expensive resistant stock and maintaining it carefully. When half your colonies die annually anyway, spending $50 per queen instead of $25 seemed wasteful. The economics of high-loss operations worked against adoption of resistance breeding.
The Virus Connection That Made Everything Worse
Understanding Varroa's impact required understanding the viral diseases the mites vectored. Initially, beekeepers focused on the mites themselves, direct parasitism, the physical damage. The virus connection emerged more slowly through research in the 1990s and 2000s.
Deformed wing virus (DWV) became the poster child for Varroa-vectored diseases. Bees infected as pupae emerged with crumpled, useless wings. They couldn't fly, couldn't forage, died within days. High DWV loads correlated perfectly with high Varroa levels. Where you found one, you found the other.
But DWV and other bee viruses existed before Varroa arrived. Testing of museum specimens and isolated bee populations revealed these viruses present at low levels in bee colonies for probably centuries. Bees had immune responses. Most infections remained asymptomatic. Colonies functioned normally despite viral presence.
Varroa transformed viral presence from background noise into epidemic disease. When a mite feeds on a bee by piercing the exoskeleton, it potentially injects viruses directly into the bee's hemolymph. This bypassed the bee's natural barriers, immune system defenses, and tolerance mechanisms. Direct injection created viral loads thousands of times higher than natural transmission through social contact or contaminated food.
Researchers documented the cascade. Mite feeds on infected bee. Mite picks up virus. Mite feeds on developing pupa in brood cell, injecting virus. Pupa develops with massive viral load. Adult bee emerges severely damaged or dies in cell. Colony weakens. More mites reproduce. Cycle accelerates.
The viral aspect explained why colonies with moderate mite levels sometimes collapsed suddenly. The mite count might look manageable, but if those mites were heavily virus-loaded and targeting brood cells, they could crash the colony through viral transmission faster than mite reproduction alone would predict.
It also explained the Colony Collapse Disorder symptoms observed in the mid-2000s. While CCD involved multiple stressors, Varroa-vectored viruses appeared in nearly every collapsed colony. The mites created conditions where viral diseases could overwhelm bee immunity, contributing to the rapid colony failures that characterized the CCD period.
Testing for viruses became another monitoring task. The presence of DWV in colony samples indicated both mite problems and future colony health risks. High viral loads predicted winter losses better than mite counts alone. Combined monitoring (mites plus viruses) gave clearer pictures of colony health than either measurement separately.
The virus connection meant that even relatively successful mite control couldn't fully protect colonies. If mites introduced high viral loads before treatment reduced mite populations, the viral damage persisted. Bees already infected didn't recover just because you killed the mites. The colony still faced immune challenges and shortened bee lifespans from pre-existing infections.
This drove the emphasis on keeping mite levels low throughout the season rather than waiting until populations exploded before treating. Prevention of viral transmission mattered more than cure. Once viruses established in colonies at high levels, recovery became difficult regardless of subsequent mite control.
How Costs Restructured the Beekeeping Industry
The economics of beekeeping transformed completely after Varroa. Operations that were marginally profitable became money-losing. Hobbyists who kept a few hives for honey found themselves spending more on treatments than they earned from honey sales. Commercial operations restructured entirely around pollination services rather than honey production.
Treatment costs added $15-50 per colony annually depending on product choice and application frequency. Monitoring equipment costs, from simple alcohol wash jars ($10) to sophisticated mite counters ($200+), represented upfront investments. Knowledge costs, learning to recognize mite damage and time treatments correctly, came through expensive mistakes and lost colonies.
Colony replacement costs escalated dramatically. Before Varroa, annual losses of 10-15% meant replacing relatively few colonies. After Varroa, with losses regularly exceeding 30-50% annually, replacement became a major expense. Package bees cost $150-180. Nucleus colonies cost $200-250. Queens alone cost $25-50.
A commercial operation with 1,000 colonies losing 40% annually faced replacing 400 colonies. At $180 per package, that's $72,000 just for replacement stock. Add treatment costs of $30 per colony ($30,000), transportation costs, labor, and equipment, and the operation needed to generate substantial revenue just to break even.
Pollination contracts provided that revenue. Almond pollination fees rose from $40-50 per hive in the 1990s to $150-200+ by the 2020s. Other crop pollination paid less but still more than honey alone generated. Commercial beekeepers shifted from honey producers who did some pollination to pollination service providers who harvested honey as a secondary income stream.
The shift created operational changes. Colonies managed for pollination needed different schedules than colonies managed for honey. Pollination contracts required hives at specific locations on specific dates, regardless of whether that timing optimized honey production. Operations maintained colonies in agricultural areas rather than seeking optimal honey flow locations.
Hobbyist beekeepers faced different economics. Without access to pollination contracts, honey sales and personal use provided the only revenue or value. Treatment and replacement costs often exceeded the value of honey produced. Many hobbyists quit. Others accepted beekeeping as an expensive hobby rather than a productive sideline.
The industry consolidated. Small commercial operations (50-200 colonies) struggled to generate economies of scale needed to absorb Varroa costs. Very large operations (2,000+ colonies) could employ full-time beekeepers, maintain treatment equipment, and negotiate better prices for supplies and pollination contracts. The middle ground became economically challenging.
Beekeeping changed from something you could profitably do part-time to requiring either full-time commitment and scale or acceptance of negative economics. The barrier to entry increased. Starting a beekeeping operation required not just knowledge of bee biology and honey production but understanding of mite monitoring, treatment protocols, disease recognition, and pollination services economics.
The Treatment-Free Movement and Its Harsh Lessons
Despite clear evidence that untreated colonies died from Varroa, a vocal segment of the beekeeping community rejected treatments entirely. The treatment-free movement combined philosophy, economics, and legitimate frustration with the chemical treadmill into an approach that accepted massive losses as the price of natural selection.
The logic had appeal. Honeybees existed for millions of years without human intervention. Feral colonies still survived in the wild. If beekeepers just stopped treating and let nature select for resistance, the problem would solve itself. Weak genetics would die. Strong genetics would persist. Eventually, Varroa-resistant bees would emerge.
The philosophy aligned with broader trends toward organic agriculture, natural systems, and rejection of chemical interventions. Treatment-free beekeeping marketed itself as more ethical, more sustainable, more aligned with bee biology than conventional management. Advocates accumulated followers, wrote books, gave talks, and created a movement.
The results were consistently catastrophic. Treatment-free operations routinely experienced 60-80% annual losses. Some years approached 90%. The survivors rebuilt slowly, only to experience similar losses the following year. Most treatment-free beekeepers gave up within 3-5 years, either returning to treatments or leaving beekeeping entirely.
A few treatment-free operations persisted longer by operating in unique conditions. Isolated locations far from other beekeeping minimized mite drift from neighboring colonies. Small-scale operations (5-10 hives) could rebuild from losses without financial ruin. Operations focusing on swarm capture from feral populations rather than buying packages avoided replacing genetics that might lack resistance.
The Arnot Forest study, often cited by treatment-free advocates, showed that feral colonies could persist with Varroa. But the study also documented that persistence came after 90%+ population crashes killed most colonies. The survivors represented a tiny fraction of the original population, maintained small colony sizes, and showed different characteristics than productive managed colonies.
The ethical arguments for treatment-free beekeeping ran into practical problems. Was it ethical to let colonies die from preventable parasitism? Did "natural selection" justify maintaining parasite-riddled bees that could serve as mite sources for neighboring operations? Treatment-free beekeepers operating near other beekeepers created conflict when their high-mite colonies spread parasites to neighbors through bee drift and robbing.
Some treatment-free advocates argued that losses weren't actually losses if you accepted them as part of the process. If you started each spring with 10 colonies, lost 7 to Varroa, but split the 3 survivors to rebuild to 6, then overwintered those to have 2 survive, and repeated the cycle, you were "keeping bees" even if colony continuity was minimal. This redefined beekeeping as an ongoing exercise in replacement rather than sustainable colony management.
The movement fractured as results accumulated. Hard-line treatment-free advocates maintained their positions despite losses. Moderate adherents adopted "treatment-free except when necessary" approaches that functioned as delayed conventional treatment. Most quietly returned to standard mite management after discovering that ideology didn't prevent colony mortality.
What the treatment-free movement revealed was that natural selection for Varroa resistance requires longer timeframes than most beekeepers could sustain. Evolution works, but slowly. The Russian bees that showed resistance came from populations that had 50+ years of mite exposure. Expecting similar resistance to develop in 5-10 years of treatment-free beekeeping ignored how evolution actually operates.
Where Native Bees Fit Into This Story
The Varroa crisis created an unexpected benefit for native bee populations. As honeybee colony density crashed from feral colony die-offs, native bees lost significant competition for floral resources. Researchers and naturalists noticed increases in bumblebee numbers, mining bee populations, and other native species in areas where honeybees previously dominated.
Varroa doesn't infest native bees. Occasional mites might climb onto bumblebees or other species, but they can't complete their life cycle. Varroa is specifically adapted to honeybee biology, requiring the precise timing of honeybee brood development for reproduction. Other bees develop faster or slower, nest differently, or have different colony structures that make them unsuitable hosts.
This created a natural experiment. Before Varroa, feral honeybee colonies occupied tree cavities, wall voids, and other nest sites across North America. Their foraging reduced flower availability for native bees. After Varroa eliminated most feral colonies, those nest sites and floral resources became available for native species.
Studies tracking pollinator communities before and after Varroa arrival found shifts. Areas that previously hosted dense feral honeybee populations showed increases in native bee diversity and abundance. The honeybee crash released competitive pressure, allowing native species to expand into ecological space they'd been excluded from.
This doesn't mean Varroa was good for ecosystems. The loss of pollinator abundance from honeybee crashes meant less total pollination in many areas. But native bee increases partially compensated for honeybee losses, and in some contexts, native bees provided more effective pollination services for native plants and certain crops.
The Varroa impact on native bees remains complex and regionally variable. In agricultural areas where managed honeybee colonies continued operating through intensive treatment, native bees gained little competitive advantage. In suburban and wildland areas where feral colonies disappeared without managed replacement, native bee responses varied based on available habitat and floral resources.
Some conservationists viewed the honeybee decline as opportunity for native bee recovery. If fewer honeybees meant more resources for natives, perhaps pollinator conservation should focus on supporting native species rather than trying to maintain honeybee populations at pre-Varroa levels. This argument created tension with beekeeping communities who viewed honeybees as agricultural necessities regardless of native bee considerations.
The practical reality is that agriculture depends on managed honeybees for pollination services at scales that native bees can't provide. Almond production alone requires roughly 2 million colonies trucked to California each February. No combination of native bee populations could replace that pollination capacity. Native bees provide important services, but they don't substitute for managed honeybees in intensive agriculture.
The Perspective From Nearly Four Decades Later
Varroa arrived in 1987. By 2025, nearly four decades of beekeeping occurred under mite pressure. What once seemed like a crisis requiring urgent solution became the permanent condition of modern beekeeping. The question shifted from "how do we eliminate Varroa?" to "how do we maintain colonies despite Varroa?"
Treatment protocols refined through trial and error. Most successful beekeepers developed regional adaptations based on local climate, mite pressures, and available resources. Northern operations focused on getting colonies through winter with low mite loads. Southern operations dealt with year-round mite reproduction requiring different timing. Everyone monitored, treated, and accepted losses as the operational cost of keeping bees.
Resistance breeding programs made gradual progress. Bees in 2025 showed better tolerance than bees in 1995, though not enough to eliminate treatment requirements. The genetics improved slowly through continuous selection, producing populations that required less intensive intervention than unselected stock. Complete resistance remained elusive, but partial improvements helped.
The chemical treadmill continued, though more sophisticated. New treatments appeared periodically. Mites developed resistance to established products. Rotation strategies evolved. Organic acids gained market share. The arsenal expanded without producing the definitive solution everyone wanted. Treatment became indefinite management rather than temporary crisis response.
Colony losses stabilized at elevated baselines. The 10-15% annual losses of pre-Varroa beekeeping never returned. Instead, 30-40% losses became the new normal through the 2010s, with periodic spikes to 50-60% during bad years. The industry adapted by breeding more queens, splitting more colonies, and accepting high replacement rates as permanent features.
The 2024-2025 season's record losses (55.6% overall, 62% for commercial operations in some surveys) suggested that even the elevated baseline wasn't stable. Whether this represented a temporary spike or new escalation in the ongoing Varroa crisis remained unclear. The industry faced the same question it faced in 1987: how do we maintain bee populations against a parasite that fundamentally wants to kill them?
Beekeeping in 2025 bore little resemblance to beekeeping in 1985. The scale changed, the economics restructured, the practices transformed, the costs increased, the knowledge requirements expanded. Someone who kept bees successfully before Varroa would find modern beekeeping almost unrecognizable. The hives look similar. Everything else changed.
The Varroa lesson extends beyond beekeeping. It demonstrates what happens when an invasive parasite encounters a naive host without evolutionary defenses. It shows how quickly biological systems can destabilize when key pressures change. It reveals the limitations of both chemical solutions and natural selection as responses to catastrophic parasite pressure.
Nearly four decades after that September discovery in Florida, Varroa still defines modern beekeeping. The mite won the first round by forcing permanent changes to beekeeping practices. The second round continues, with beekeepers adapting, mites evolving, and both locked into an ongoing biological arms race with no end visible.
The small reddish-brown parasite, barely visible without magnification, restructured an entire agricultural industry. That's the Varroa legacy. Not extinction, which beekeepers prevented through constant intervention. But permanent transformation of how humans maintain honeybees in the modern world.